Cortellis Clinical Trials Intelligence
Position your clinical trials for success from day one
Optimize clinical trial planning and increase your chances of success
Confidently plan and design clinical trials by reducing the time spent compiling and analyzing protocols, sites, outcomes, endpoints and more.
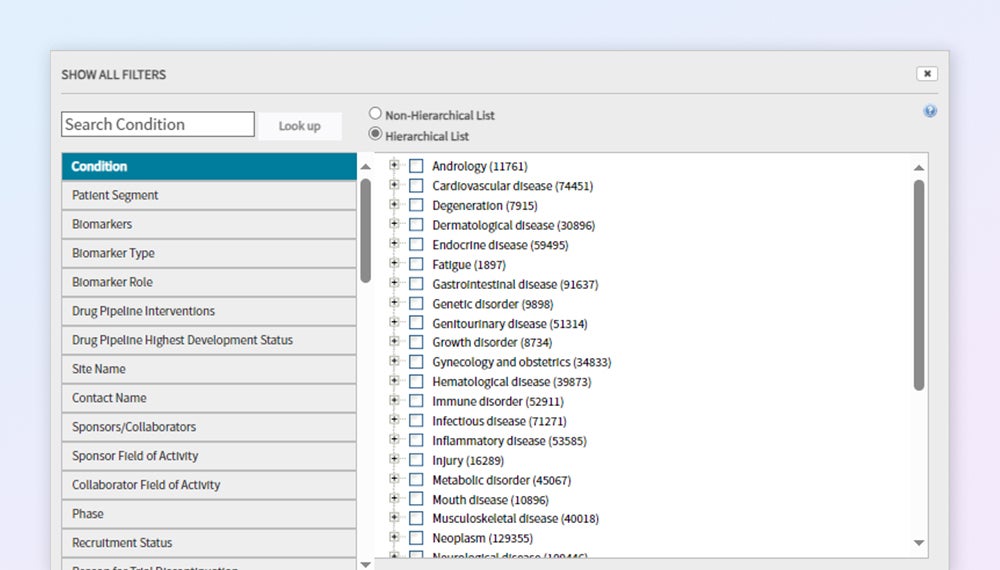
Design your ideal protocol the first time
Plan trial protocols and choose appropriate endpoints using reliable clinical trials data curated by experienced industry analysts.
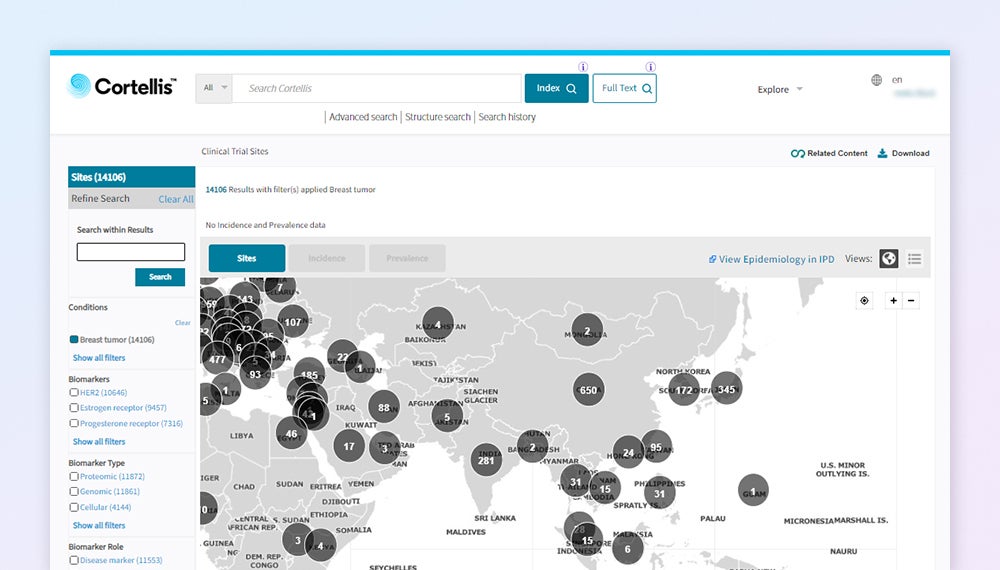
Pinpoint experienced sites that have access to your patients
Identify reliable and experienced clinical trial sites based on their success recruiting patients in your targeted populations.
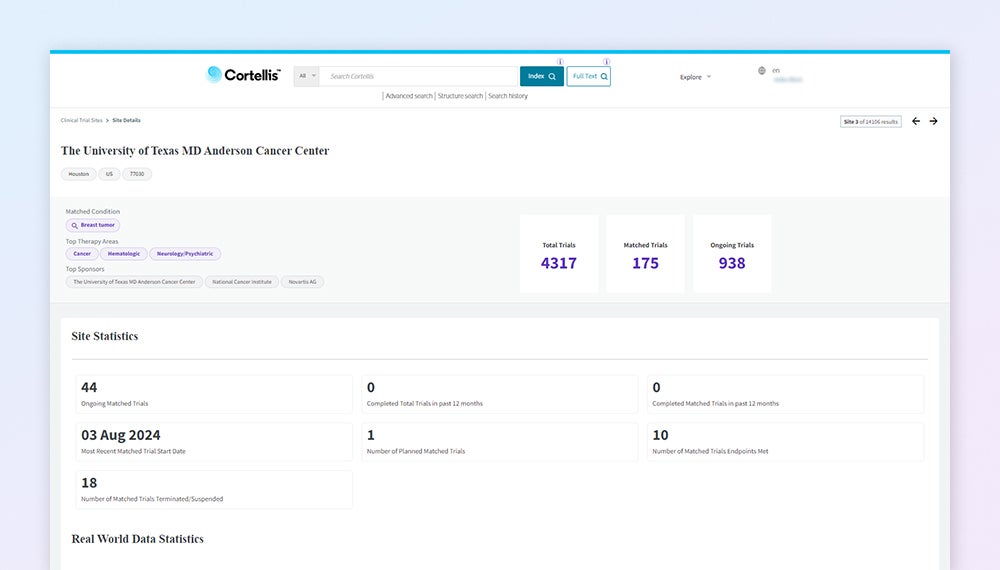
Perform thorough trial feasibility analysis
Understand successful protocols, sites, and patient segments by evaluating extensive site intel that integrates epidemiology data, site availability and recruitment.
Accelerate clinical development decisions
Find answers within seconds
Fully searchable and indexed clinical trial content covering over 600k+ global trials.
Comprehensive disease coverage
Robust coverage of clinical trials for 30+ therapy areas, including rare disease and orphan drug trial data not found elsewhere.
Expert team
Access our product specialists and subject matter experts through live chat and 24/5 support service.
Transformative clinical trials intelligence
Pinpoint your ideal site
End-to-end clinical operations workflow to identify, select and analyze key sites of interest. Covering 277k+ sites across 200 countries with integrated U.S. claims data and epidemiology insights.
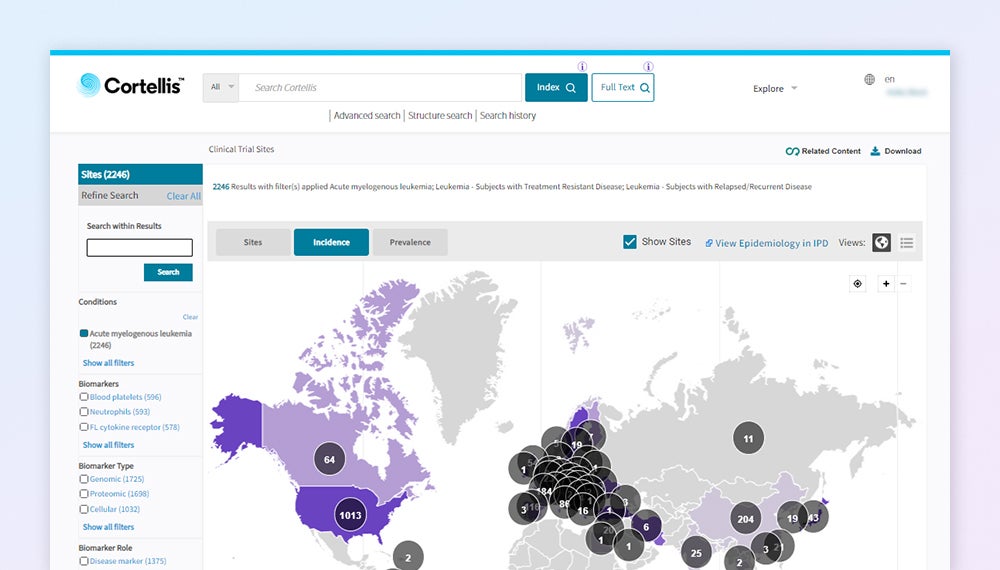
Track competitive trial strategies in your therapy area
Explore competing trials by disease area, patient segment, trial design, recruitment status, and mechanism of action. Find where trials are running and expected end dates and plan your trials better using trials that met their endpoint versus those that did not.
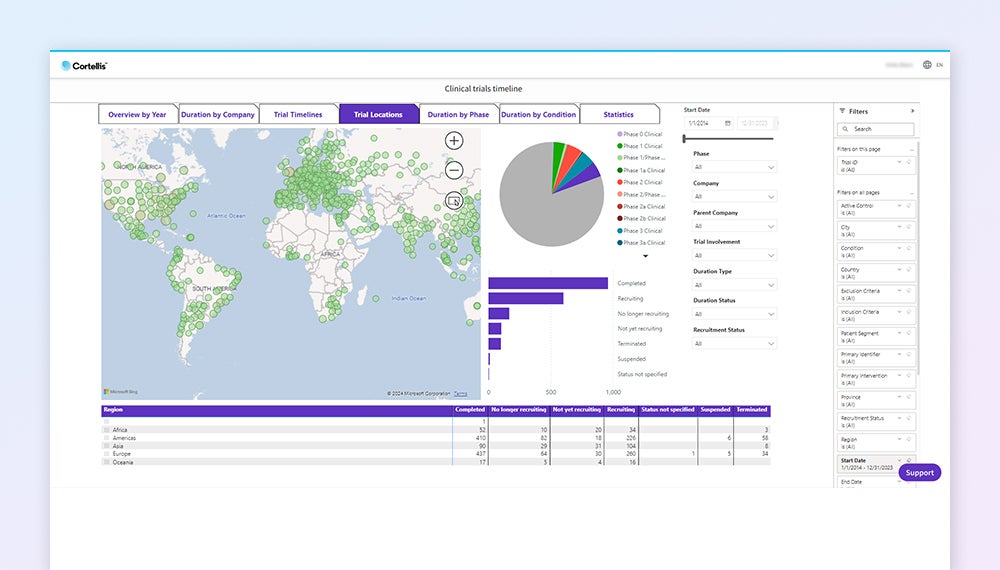
Position your trials for success
Benchmark your trials to timelines and endpoints by tracking key milestones including primary endpoint completion and enrollment dates. Understand all endpoints reported for a trial and analyze what led to trial success or failure.
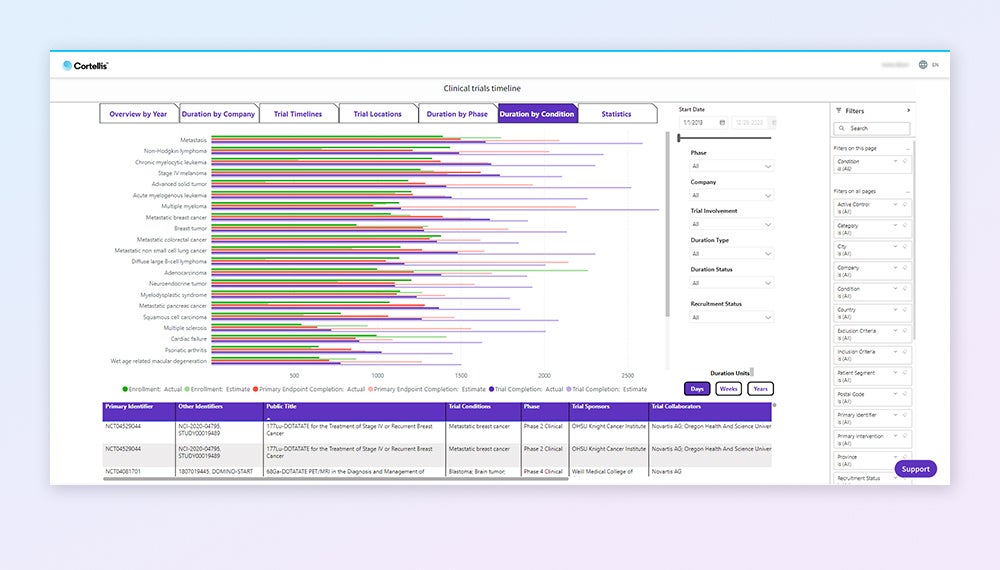
Explore protocol details
Access comprehensive clinical trial reports, including protocol & results, inclusion / exclusion criteria, trial arms & measurements, sites, biomarkers, devices and more.
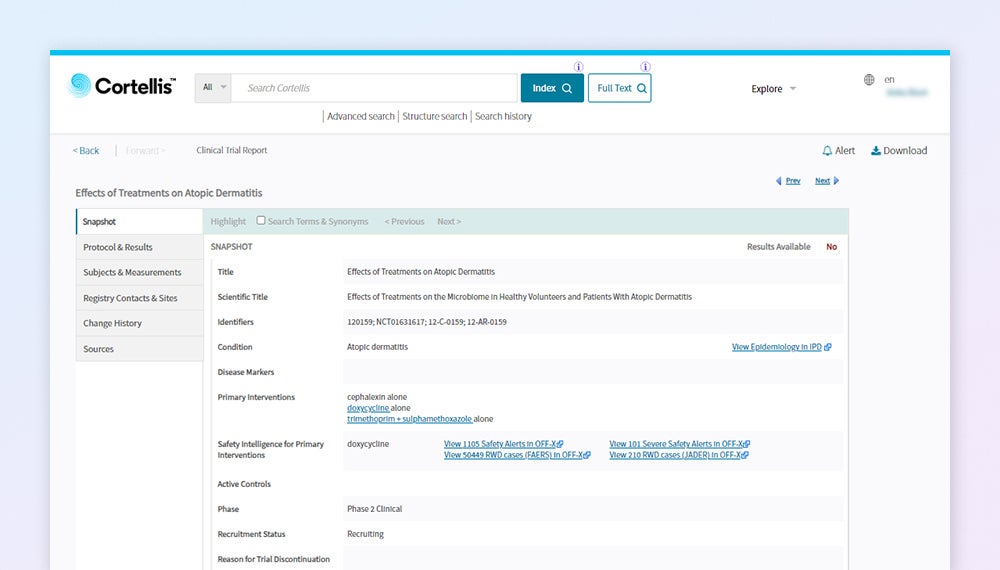
Want to learn more?
Contact us to schedule a demo of Cortellis Clinical Trials Intelligence.
600k+
813k+
89k+
46k+
Resources
Cortellis allows the clinical operations team to validate what their CRO is telling them so that they can have more informed discussions around site performance and enrollment strategy in the rare disease space.
Biopharma company focused on rare diseases
Cortellis helps the clinical operations team feel more confident in making decisions around site selection.
Information professional Global biotech company
Cortellis provides us the coverage in the rare disease space we were looking for. The content enabled enrollment projections at the site level using the built-in analytics and enrollment data.
Information professional Global biotech company
The Trial Timeline viewer is particularly popular, enabling us to quickly generate graphics that can be shared with senior management. We are also pleased that the Clarivate team has been adding enrollment periods and rates since Q1 2017 and impressed by the volume of trials curated to date.
US-based biopharma company
Successfully translate drugs from the lab to the clinic.
Fraud Warning
Please be advised that recently there have been fraudulent job offers and interviews using the Clarivate name, logo and even names of our colleagues.
Please be aware that Clarivate will:
- Never ask for payment of any kind as part of our hiring or onboarding processes
- Never ask an applicant to email sensitive personal information, such as a Social Security Number, birthdate, credit card or bank account information
- Never issue pre-employment checks to purchase office supplies
- Never ask you to pay up for an external course and upskill
If you have any question about a position posted in our company name, please check our current open positions on the Clarivate website Careers pages or contact one of our recruiting team members directly.
If you have been the victim of a scam, please contact your local law enforcement agency.
Federal Transparency In Coverage Rule
This link leads to the machine-readable files that are made available in response to the federal Transparency in Coverage Rule and includes negotiated service rates and out-of-network allowed amounts between health plans and healthcare providers. The machine-readable files are formatted to allow researchers, regulators, and application developers to more easily access and analyze data.





